Effect of DIRECT transfer to ANGIOsuite on functional outcome in patient with severe acute stroke treated with thrombectomy

Why the DIRECT ANGIO study?
Problematic
Stroke remains a large cause of death and disability in France and in the world. 1 patient present a stroke every 3 minutes and 75% of them will not be independent at 3 months. Acute ischemic stroke due to the large vessel occlusion (LVO) of the anterior circulation is the most severe form and represent 30% of overall strokes. Endovascular mechanical thrombectomy, in association or not with intravenous thrombolysis is the standard of care for patients with acute ischemic stroke and anterior LVO since 2015.
 |
 |
 |
 |
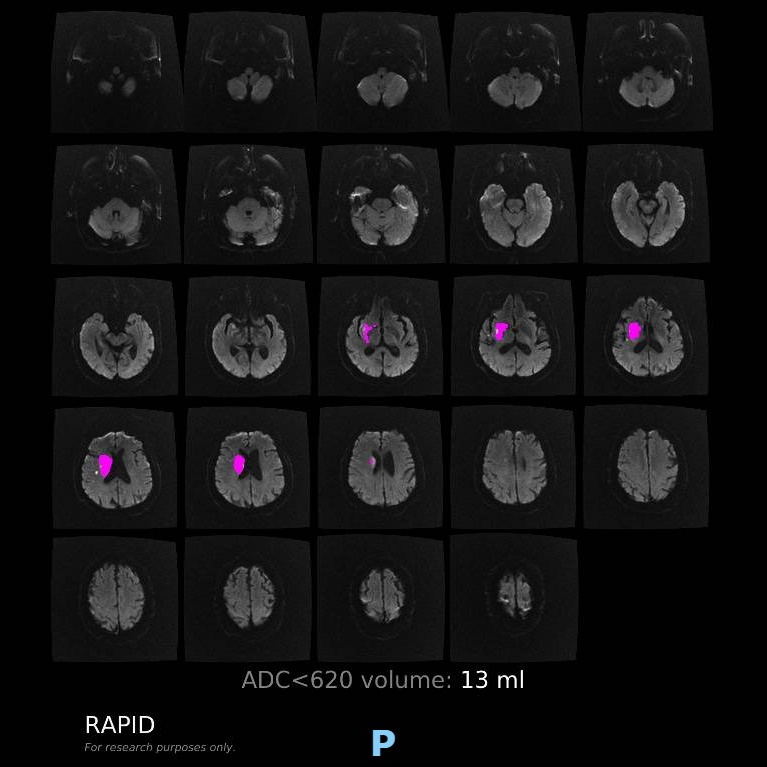 |
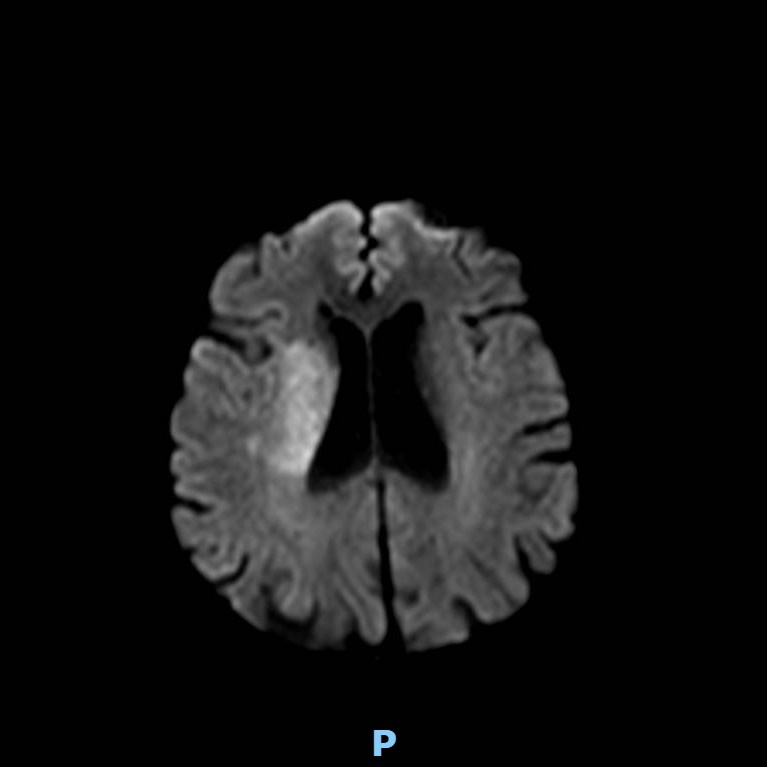
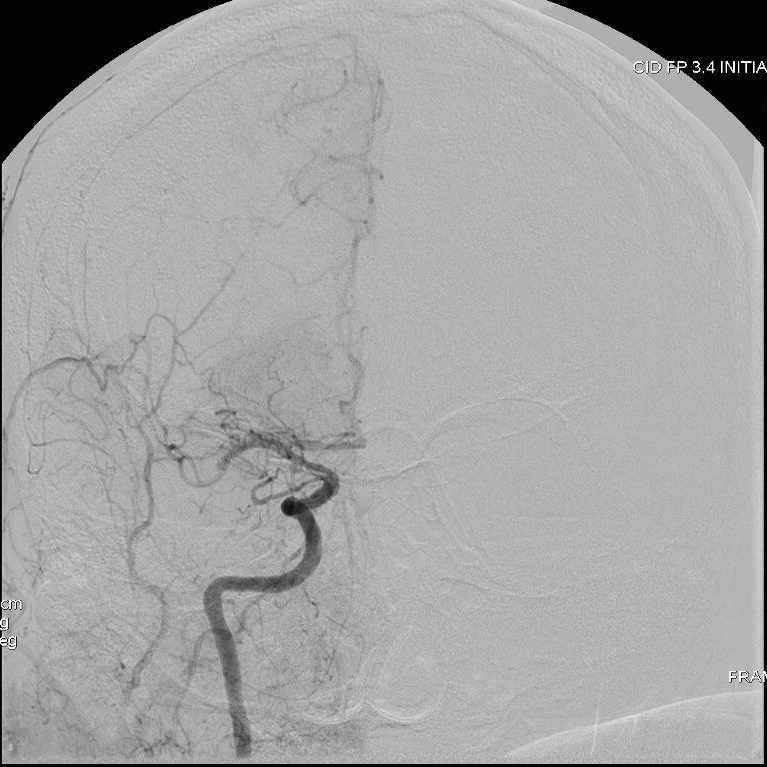
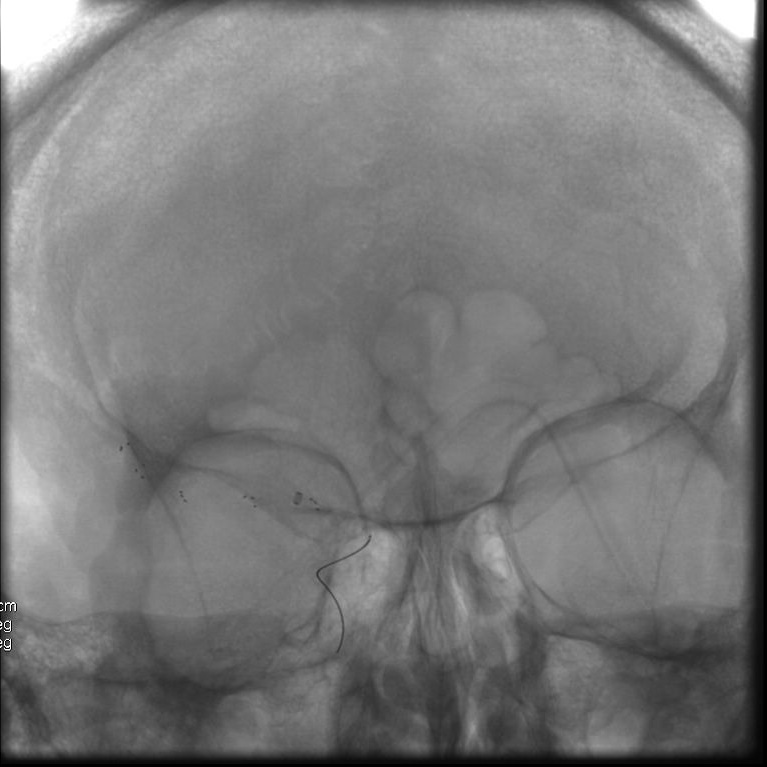
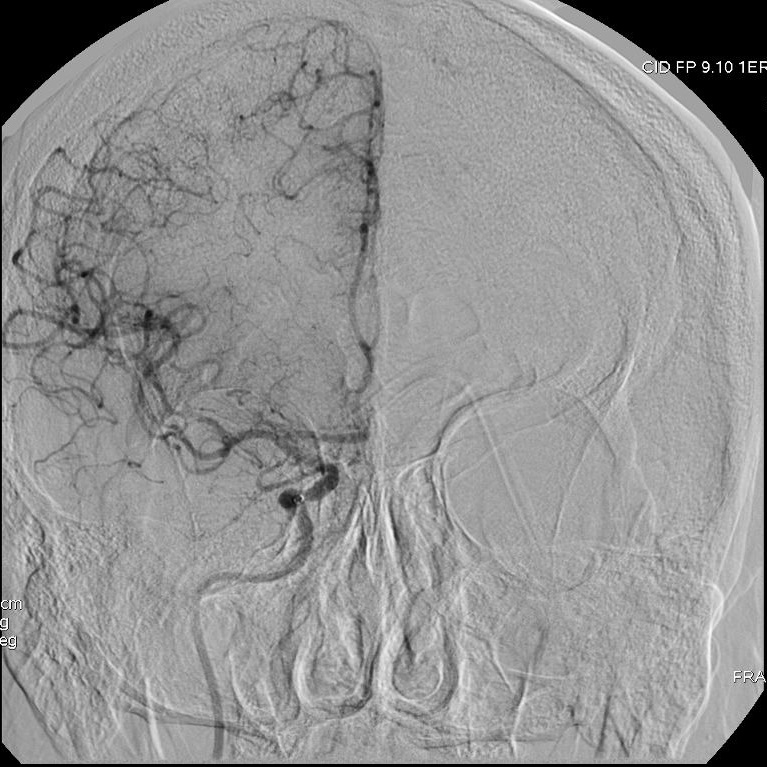
Patient presenting an acute ischemic stroke due to proximal anterior circulation occlusion. Intravenous thrombolysis and endovascular thrombectomy showing the arterial proximal occlusion. Early successful reperfusion allowing the extension of the ischemic core and the disability.
Context
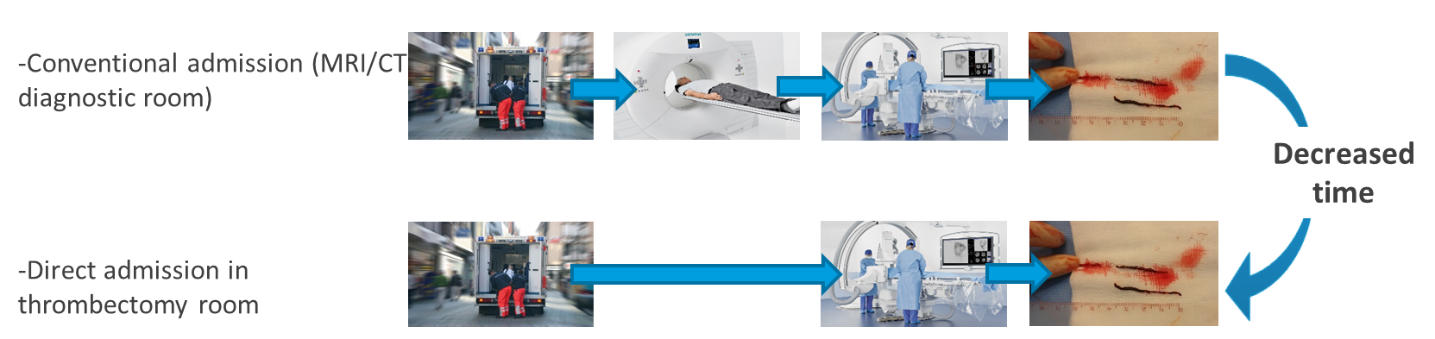
Endovascular mechanical thrombectomy increases functional independence in patients with acute ischemic stroke caused by anterior circulation large vessel occlusion, and the probability to achieve functional independence decreases by 10% for each 30 minutes delay to reperfusion. Therefore, it is currently crucial to achieve fast triage and initiation of endovascular therapy. To date, patients with a suspected stroke perform first an imaging and secondary transfer to the angiosuite for mechanical thrombectomy if large vessel occlusion is confirmed. This approach results in prolonged delays in delivering definitive therapy in the setting of large vessel occlusion strokes, whereas the angiosuite has imaging facilities to rule out intracranial haemorrhage with the cone beam CT and confirm proximal arterial occlusion by catheter angiogram.
A solution?
Direct admission to the thrombectomy room (without going through MRIMRI means Magnetic Resonance Imaging but it can also mean the exam, the image, or the imager. Further information on MRI and its technique More or CT) to reduce intra-hospital management delays, and consequently the delay of cerebral reperfusion.
Therefore, we aim to investigate whether direct angiosuite transfer (DAT) is superior to standard imaging/emergency department-based management in achieving 90-day functional independence in patients presenting with an acute severe neurological deficit likely due to LVO and requiring emergent treatment with MT.
How is the study organized?
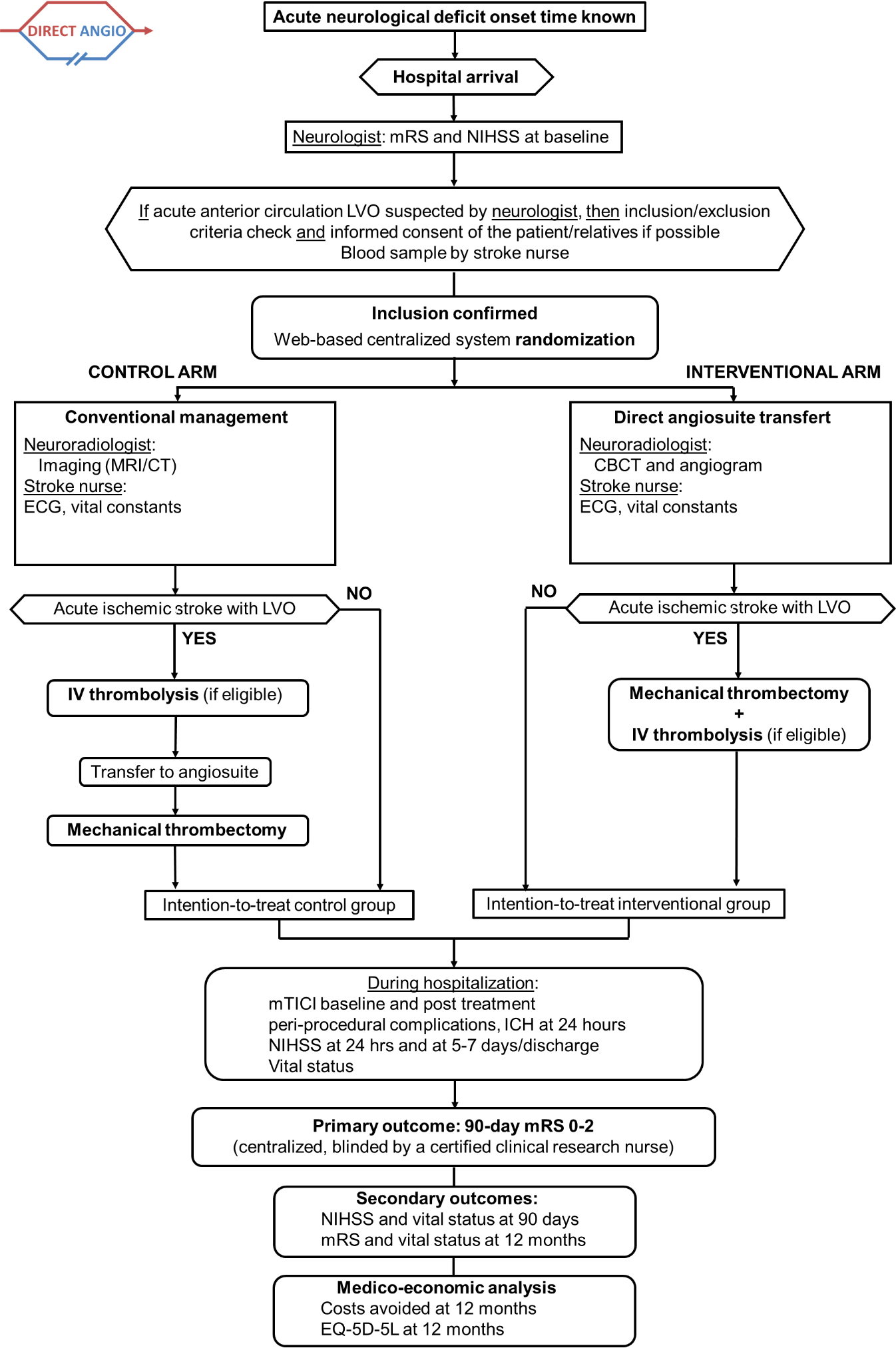
The study is running in 13 French comprehensive stroke centres to enroll 208 patients overall. On arrival in angiosuite and after rapid neurological examination (using National Institutes of Health Stroke Scale (NIHSS) and mRS scores) and blood sample, the patient undergoes CBCT in order to exclude non-ischaemic stroke and angiogram to confirm LVO.
After inclusion, the patients are randomised in two arms using a web-based centralised system with a 1:1 ratio to either direct angiosuite transfer (DAT) or standard management.
Primary objective
Participant inclusion and exclusion criteria
Inclusion criteria
- Between 18 and 85 years,
- Rapid neurological examination by the neurologist of the participating centre before randomization,
- Only mothership admissions,
- Acute severe neurological deficit at hospital admission confirmed by neurologist defined as:
-
- Unilateral motor deficit with a total score ≥ 5
- Facial palsy (item 4 NIHSS 0 to 2)
- Arm (item 5 NIHSS 0 to 4)
- Leg (item 6 NIHSS 0 to 4)
- Unilateral motor deficit with a total score ≥ 5
- AND
- Cortical symptom with a total score ≥ 1
- Language (item 9 NIHSS 0 to 3)
- Extinction (item 11 NIHSS 0 to 2)
- Cortical symptom with a total score ≥ 1
-
- Prestroke modified Rankin Scale score 0 to 2,
- Hospital admission ≤ 5hours,
- Immediate availability of the angioroom and endovascular treatment team at the randomization,
- Affiliation to/beneficiary of a social regime.
Exclusion criteria
- Severe allergy to contrast agents,
- Any terminal illness such that patient would not be expected to survive more than 90 days,
- Pregnant or breastfeeding women,
- Consent refusal or opposition of the relatives,
- Under legal protection.
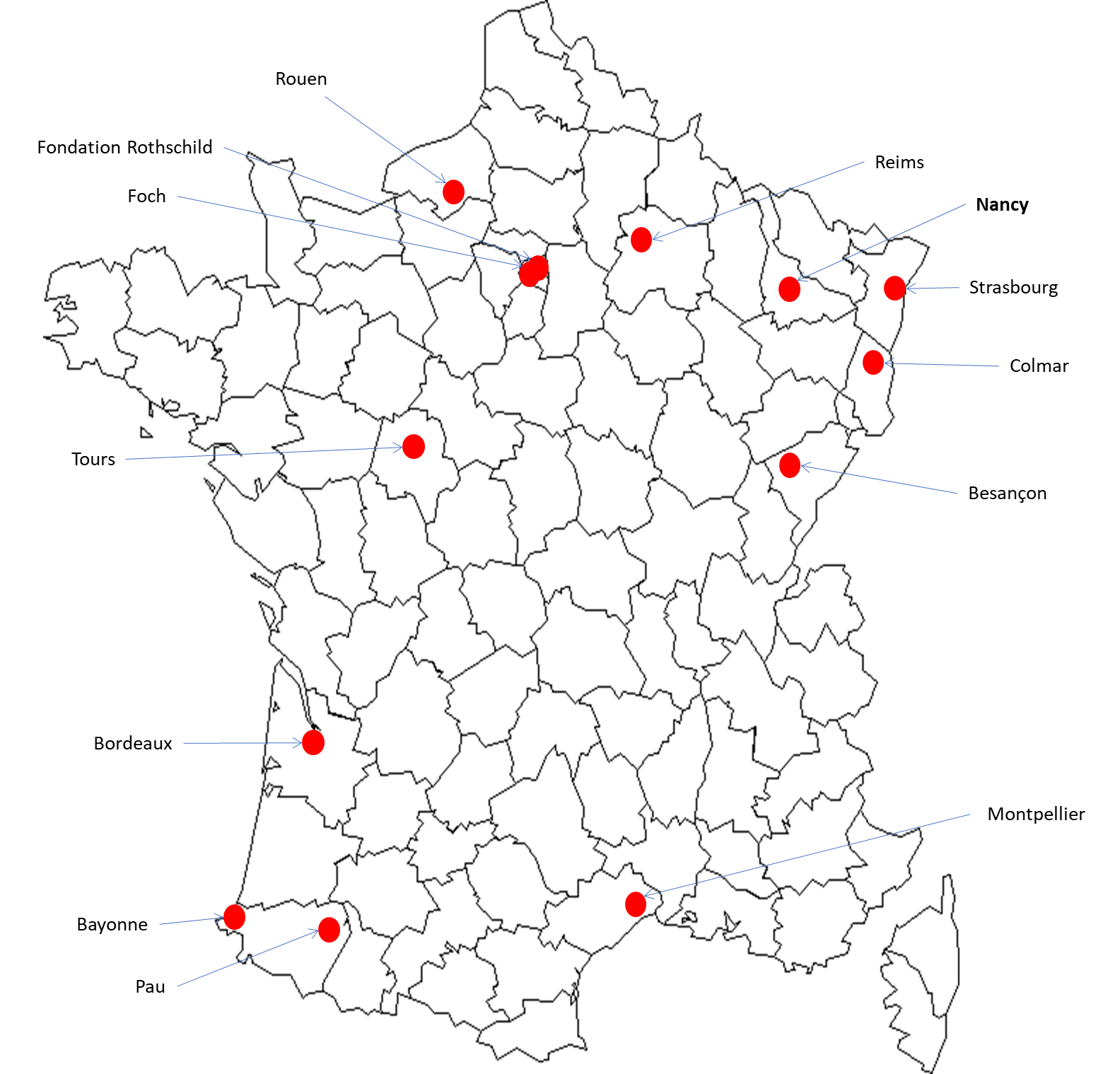
Participating centres
Sponsor & Funding
DIRECT ANGIO promotor is the Hostpital of Nancy, France.
The study is financed by the General Direction of Healtcare (DGOSAu sein du ministère chargé de la santé, la direction générale de l’offre de soins (DGOS) se substitue depuis le 16 mars 2010 à la direction de l’hospitalisation et de l’organisation des soins (DHOS). "L’innovation médicale, qu’elle soit technique (acte, dispositif médical, médicament) ou organisationnelle, est une composante incontournable de la qualité des soins et de la performance de l’offre de soins. Grâce aux différents outils dont elle dispose, la DGOS prend en charge l’innovation médicale :
en assurant le financement adapté de la validation des innovations médicales
en facilitant leur évaluation
en permettant leur diffusion optimale aux patients
tout en coordonnant son action avec celles des autres partenaires institutionnels."
Pour en savoir + More) in the 2018 interregional programe (PHRC-i, GIRCI Est) with the amount of 295.990 euros.
In 2021, a donation of €25,000 was obtained from Medtronic.
In 2022: a donation of €5,000 was obtained from MIVI Science
More information about the study
Registration on ClinicalTrials.gov (NCT03969511): https://clinicaltrials.gov
Publication of the protocolIn clinical research, a protocol is a document where one can have a complete description of a given investigation: purposes, way it will be conducted, number of subjects included, contributors, statistics aspects, etc. It is an essential document dated and approved both by the sponsor and the investigator. It is a base for the competent authority to decide to implement a study or not. All the contributors must observe it. More in BMJ Open: https://bmjopen.bmj.com
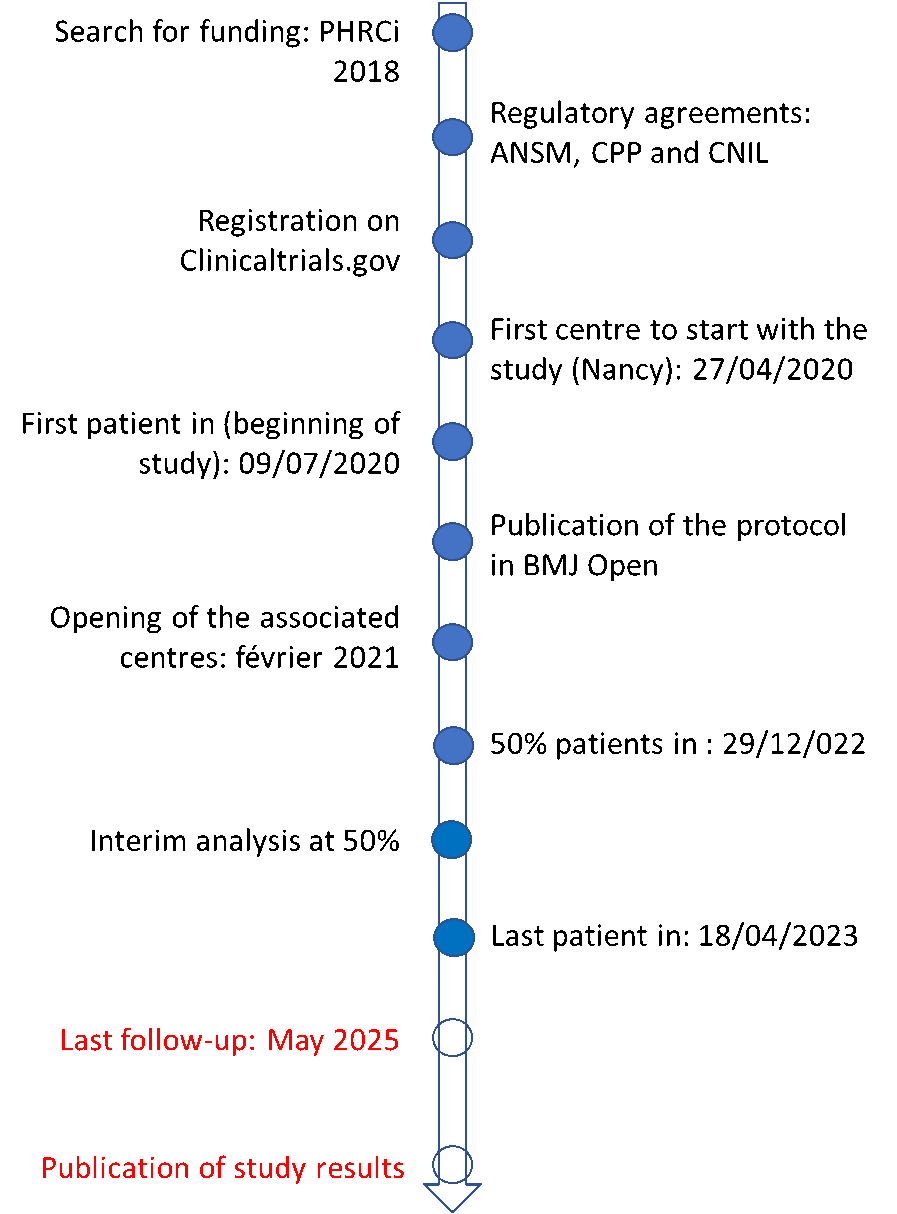
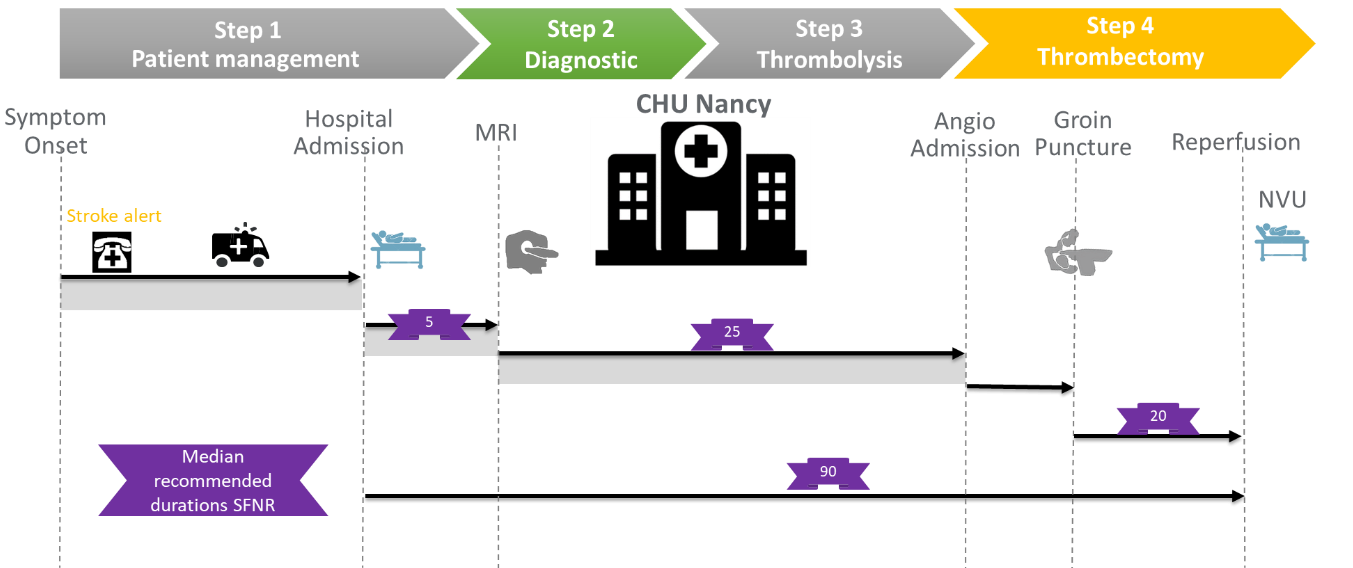
Milestones of DIRECT ANGIO study
Contacts
Coordinating Investigator
Pr. Benjamin GORY
Adress : Service de Neuroradiologie Diagnostique et Thérapeutique, CHRU Nancy – Hôpital Central,
29 Avenue du Maréchal de Lattre de Tassigny, 54035 Nancy
Mail :
Phone : +33 3 83 85 95 27
Referring neurologist
Pr. Sébastien RICHARD
Adress : Service de Neurologie, CHRU Nancy – Hôpital Central,
29 Avenue du Maréchal de Lattre de Tassigny, 54035 NANCY
Mail :
Phone : +33 3 83 85 21 12
Clinical Study Coodinator
Aboubaker CHERIFI
Adress : CIC-IT Nancy – DRCI, CHRU Nancy – Hôpitaux de Brabois, Bâtiment Recherche, RdC,
rue du Morvan, 54511 VANDOEUVRE-LES-NANCY
Mail :
Phone : +33 3 83 15 70 84
Research Vigilence
Dr. Nadine PETITPAIN / Dr. Marie-Lauren ANTOINE
Adress : Centre Régional de Pharmacovigilance de Lorraine, CHRU Nancy – Bâtiment de Biologie et de Biopathologie – Hôpitaux de Brabois,
rue du Morvan, 54511 VANDOEUVRE-LES-NANCY
Mail :
Phone : +33 3 83 65 60 90 / 60 91